Focal Segmental Glomerulosclerosis
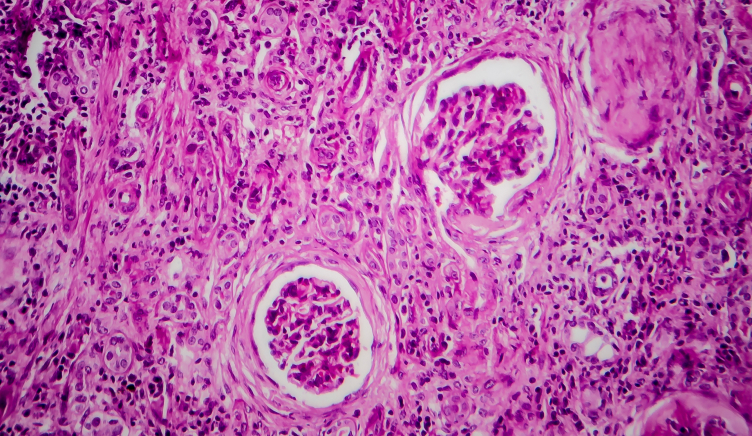
Focal segmental glomerulosclerosis (FSGS) is one of the most common causes of primary glomerular disease in adults. It is a chronic pathological process caused by injury in the glomeruli of the kidney; the glomeruli are essential functional units which perform the first step in the filtration of blood and generation of urine, hence inflammation and fibrosis in these tissues result in decline in kidney function.
Fibrosis is a major clinical component of FSGS, due to the development of scar tissue around the glomeruli. If unchecked, the continued injury to the glomeruli causes progression to nephrotic syndrome and ultimately to end-stage renal failure (ESRD). Typically, the time from the onset of gross proteinuria to ESRD is 6-8 years.
For ESRD patients who successfully undergo kidney transplantation, FSGS recurs post-transplant in approximately a third of cases and leads to a 5x higher risk of graft (new organ) loss.
Clinical standard of care focuses on treatment to control hyperglycaemia and hypertension with angiotensin-converting enzyme (ACE) inhibitors, angiotensin-receptor blockers (ARBs), or other antihypertensives. Patients who are persistently nephrotic after a course of conservative therapy or who present with complications from nephrotic syndrome require more aggressive treatment with prednisone (corticosteroid) or immunosuppressive agents.
Overall, more than 50% of FSGS patients with primary disease are steroid resistant and there are no therapeutic options to address the fibrosis and disease progression arising from the continued kidney injury.
As such there is a significant need for a safe, efficacious, and well-tolerated anti-fibrotic treatment for FSGS.
source references:
Disclaimer
All of the candidate drugs described on this site are investigational products which have not received marketing authorization or approval by any regulatory agency, including the US Food and Drug Administration, the European Medicines Agency, or the Australian Therapeutic Goods Agency. The investigational drug products are undergoing clinical studies to evaluate the safety and effectiveness in humans.
